Anti-abortion advocates see promise in artificial womb technology, or AWT, for increasing the chances of survival outside of the womb for premature infants.
Questions about the ethics of such a technology arose this week after the Independent Pediatric Advisory Committee of the Food and Drug Administration met on Tuesday and Wednesday to assess whether to advise the FDA to grant an investigational device exception to devices that essentially replicate the environment of a uterus to continue the process of fetal development.
HOUSE GOP LEADERS SIGNAL POSSIBLE FUNDING BREAKTHROUGH WITH CONSERVATIVE HOLDOUTS
“The term ‘artificial wombs’ is misleading about this technology,” Catholic policy expert Leah Libresco Sargeant told the Washington Examiner. “A [neonatial intensive care unit] incubator is already a kind of artificial womb, trying to provide some of the support the baby would have otherwise gotten from his or her mother. If we’re able to provide better support to extremely premature babies, I’m all for it.”
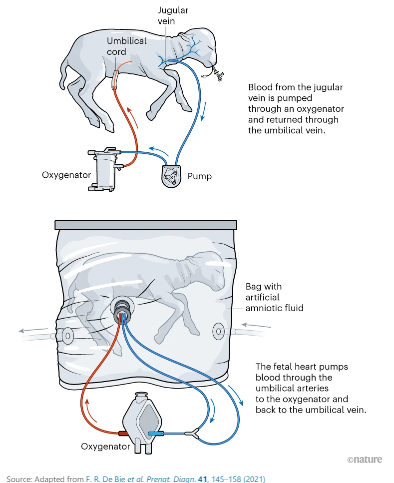
In 2017, the Children’s Hospital of Philadelphia, or CHOP, conducted an experiment that led to eight lambs successfully completing their gestation process in a bag-like apparatus with an external oxygenator circulating blood flow like a placenta. The lambs were developmentally equivalent to human fetuses at 23 weeks gestation, and they remained in the artificial womb for up to four weeks.
Two years after the experiment, several of the researchers joined the medical start-up Vitara Biomedical, which has developed the EXTEND, or the Extra-uterine Environment for Newborn Development.
“Artificial womb technology is a proposed therapeutic strategy that aims to bridge the period between extreme preterm birth and later gestation to allow for organ maturation in a system that mimics the womb environment and provides artificial placental support for nutrition and gas exchange,” according to a briefing document provided by the FDA to the Washington Examiner.
Lung capacity, organ maturity, and certain neurological functions in human fetuses develop later in pregnancy, making the risk of death or long-term health consequences of premature birth particularly high.
According to data from the FDA, 13.4 million children worldwide are born prematurely annually, accounting for 900,000 deaths per year.
In the United States, 10% of live births are of premature infants, most of whom are born after 37 weeks gestation. Still, premature birth accounts for 65% of U.S. infant mortality, with the majority of deaths each year consisting of children under 28 weeks gestation.
The idea of artificial wombs replacing women took social media by storm in December when a hyper-realistic video of a conceptualized fetus lab was mistakenly taken by many online as an existing long-term project.
Despite dystopian concerns of misuse, Libresco Sargeant said it is more important to look at their actual practical effects for the survival of premature infants.
“It’s instructive to look at how existing incubators [or] ‘artificial wombs’ are used — they support babies who can’t safely stay with their mothers; they’re a harm-minimizer, not an equivalent,” Libresco Sargeant said. “And babies who receive extensive medical support still benefit from kangaroo care when possible — they need active love and tenderness, not just fluids and monitors.”
Anti-abortion advocate Frank Pavone of Priests for Life told the Washington Examiner that he had a positive view of the future for AWTs as being able to, in the long-term,” introduce into the abortion debate a clear distinction, now, between ending the pregnancy and ending the life.”
“The viability of an unborn baby very much depends on the technology that’s waiting for them on the other side. It also depends on the willingness of the people on the other side to save their life,” said Pavone, whose strident anti-abortion activism has stirred controversy and whose online statements played in role in his being dismissed from the priesthood.
Robin Pierucci, an anti-abortion neonatologist with 25 years of clinical practice, told the Washington Examiner that although there is great promise for this technology for keeping premature and unborn patients alive, she is concerned that more data on safety and efficacy is necessary in the immediate term before use on human infants.
“Oh, it’s gonna benefit all babies eventually. That’s not a good enough reason,” said Pierucci. “We don’t use our babies as guinea pigs for the research of the common good.”
“I think we should be embracing medical technology that really keeps unborn babies safe as well as the ones who arrive prematurely,” said Pierucci. “I would love to have data that says, ‘Here’s a way to take even better care of our premature babies.’ It’s just not there yet.”
Pierucci emphasized the need for trials in non-human primates, the gold standard of medical animal testing. FDA briefing materials indicate, however, that the small size of primate fetuses at the equivalent stage of human gestation would make cardiovascular intervention tremendously difficult.
The FDA’s briefing materials also indicated that any potential for first-in-human clinical trials for these types of investigative devices would require a “robust plan for safety monitoring and adverse event reporting,” “clinically meaningful treatment” evaluation, and “effective informed consent.”
“I have parents coming to me in the unit,” said Pierucci. “I can’t tell them in good conscience that putting them on this artificial womb is safer, or a greater benefit than it is being admitted into the NICU. If we can’t say that the benefits outweigh the risks, it’s unethical to offer this.”
CLICK HERE TO READ MORE FROM THE WASHINGTON EXAMINER
“The good news is our outcomes have improved. That just demands better research,” Pierucci said.
An FDA spokesperson confirmed for the Washington Examiner that the committee has not yet issued a recommendation to the administration.